Background: Venetoclax in combination with azacitidine or low-dose cytarabine has become standard treatment for newly diagnosed elderly or unfit patients with acute myeloid leukemia (AML). Since approval of venetoclax in 2020 in Japan, venetoclax combination therapy have been also used for relapsed or refractory patients although its role in relapsed or refractory disease is not well defined. The survival impact of chemotherapy with venetoclax combination therapy compared with that without venetoclax combination therapy on AML patients in a real-life setting is scarcely reported.
Objective: The aim of this retrospective study was to evaluate chemotherapy with or without venetoclax combination therapy in patients with newly diagnosed AML in our institution.
Patients and Methods: This study included 234 non-M3 newly diagnosed AML patients who were treated with or without venetoclax combination therapy between February 2008 and July 2023 in our hospital. We evaluated overall survival and composite complete remission (complete remission or complete remission with incomplete hematologic recovery). Overall survival was compared with the use of a cox proportional-hazards regression model adjusted for age (1-year increase) and cytogenetic risk (favorable or intermediate vs. adverse). Composite complete remission was compared between the treatment groups with the use of a logistic regression model adjusted for the same prognostic factors. Subgroup analyses of overall survival was performed according to variables based on disease characteristics at baseline to assess whether the beneficial effect of venetoclax combination therapy varied among specific patient subgroups.
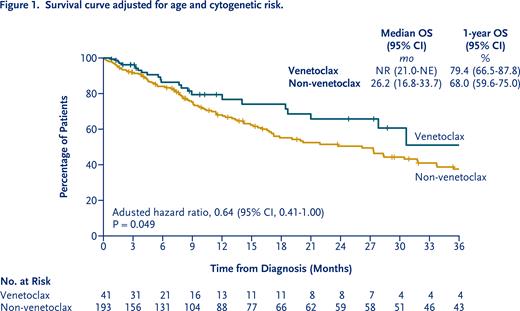
Results: We identified 41 patients who were treated with venetoclax as first-line or later treatment and 193 patients who were treated without venetoclax. In the venetoclax group, 26 (63.4%) received venetoclax combination therapy as first-line and 15 (36.6%) as second- or later-line treatement. Twenty (48.8%) were treated with venetoclax combined with azacitidine, 12 (29.3%) with low-dose cytarabine and 9 (22.0%) with both in their course of treatment. Patients in the venetoclax group was older than those in the non-venetoclax group (median age, 76 years vs. 67 years, respectively; P<0.001). FLT3, NPM1, TP53 and IDH1/2 mutational status was comparable between groups. The percentage of patients classified as poor cytogenetic risk category according to the NCCN guidelines for AML, 2021 was higher in the venetoclax group than that in the non-venetoclax group (51.2% vs. 28.0%, respectively; P=0.005). The percentage of patients who umderwent allogeneic hematopoietic stem cell transplantation was lower in the venetoclax group than that in the non-venetoclax group (2.4% vs. 16.6%, respectively; P=0.014). Composite complete remission was achieved in 46.3% (95% CI, 30.7 to 62.6) of the patients in the venetoclax group and 54.4% (95% CI, 47.1 to 61.6) of the patients in the non-venetoclax group (P=0.199). In a Cox model adjusting for age and cytogenetic risk, the 1-year overall survival rate was 79.4% (95% confidence interval [CI], 66.5 to 87.8) in the venetoclax group, as compared with 68.0% (95% CI, 59.6 to 75.0) in the non-venetoclax group (adjusted hazard ratio, 0.64; 95% CI, 0.41 to 1.00; P=0.049). Subgroup analysis of overall survival showed a greater survival benefit in the venetoclax group than in the non-venetoclax group in patients who were 70 years of age or older (P=0.003 for interaction) and in patients with de novo AML (P=0.022 for interaction) after adjustment for age and cytogenetic risk.
Conclusions: These real-world data suggested that incorporation of venetoclax combination therapy into existing therapies improved survival in patients with AML. The survival benefit of venetoclax combination therapy was greater in elderly patient and in de novo AML patients than secondary AML patients. Interpretation of the results must be careful because the sample size in the venetoclax group was small. To confirm our findings, further investigation in a larger patient population is required.
Disclosures
Usuki:Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Alnylam Japan: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Alxion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Aperis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Chugai: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daichi Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Eisai: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kyowa-Kirin: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Mundi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Nippon Shinyaku: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ohara: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ono: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Otsuka: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sando: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sumitomo-Dainippon: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; SymBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Yauklt: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal